The Results Are In: Chondrosarcoma Patient Registry Result #2 Diagnosis
The ChondrosarcomaFoundation was the first foundation to be inducted into the NORD IAMRARE Patient Registry 2.0 platform and the 44th Foundation to have a Patient Registry in the platform itself. In February 2023, we had a soft launch with a few board members participating in early February 2023 and we addressed some errors and made improvements before our official launch on March 1, 2023. Since our launch we have had over 230 CS Patients participate in the registry and we just completed our first full data analysis from the data collected from 169 participants who completed all of the surveys in the registry. This is the first global Natural History Study addressing Chondrosarcoma.
Over the next few weeks we will share major findings (one result at a time) to help both the sarcoma / oncology medical profession and the CS patient community understand and use these results towards building developing better practice / interventions as well as better research and clinical trials.
This is an on-going retrospective analysis of the Chondrosarcoma Patient Registry. The database includes demographic, medical history, diagnostic, clinical, genetic, and patient-reported measures collected from patients or their relatives worldwide (from 19 countries) via the customized survey (IAMRARE® program powered by the National Organization for Rare Diseases). Patients were stratified by sarcoma type in three cohorts – conventional CS (conventional grade 1-3 sub types), non-conventional CS (other sub types), and unknown CS (unspecified chondrosarcoma type). Outcomes were explored in the total sample and statistically compared between conventional and non-conventional CS.
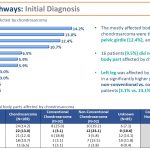
Diagnostic Pathways: The most frequent symptoms occurred in chondrosarcoma patients were pain (64.5%), lump/swelling (29.6%), and restricted mobility (23.1%). A significantly higher rate of lump/swelling was observed in non-conventional chondrosarcoma. About half of the study population (49.7%) was initially diagnosed with chondrosarcoma. Misdiagnosis was detected in 42.3% of patients with other than chondrosarcoma initial diagnoses. To detect chondrosarcoma, the most common diagnostic tools utilized were biopsy (69.2%) and MRI (67.5%), while medical professionals involved were oncologists (48.5%). Gene testing was performed in 18.9% of patients.
Conclusion: Diagnosing chondrosarcoma is difficult, The misdiagnosis rate of 42.3% is too high. Our results shows that tissue biopsy evaluated by a pathologist is the best indicator of a successful diagnosis of chondrosarcoma. Early detection also needs improvement. Pain, a lump, swelling and increased resticted mobility are early indicators and should be examined as soon as possible.
Your participation is essential. Be A Part of the Chondrosarcoma Patient Registry.
To Register: https://chondrosarcoma.iamrare.org/
It is not too late to sign up to participate in the Chondrosarcoma Patient Registry. The register is open at any time for CS Patients or Caregivers to go on-line and complete 9 surveys which will take less than an hour to complete. We need you to share your journey and help progress research in chondrosarcoma.
The Chondrosarcoma Patient Registry creates a platform for patients around the world to strengthen their voices and share information about this rare bone cancer. This global resource will provide data for researchers to use to advance drug development and treatment options and help improve patient care. The patient information that the registry collects will help give direction to scientists on areas in which to focus their research efforts, and to hopefully allow clinicians to see trends in patient responses to treatment for chondrosarcoma. The hope for the future is to illuminate treatments that have had some success that may become the standard of care for this rare bone cancer.