More Self-reports are needed for Prescribed or Experimental Medications
The Chondrosarcoma Foundation was the first foundation to be inducted into the NORD IAMRARE Patient Registry 2.0 platform. Since our launch on March 1, 2023, we have had over 230 CS Patients participate in the registry and we are reporting the results of our first full data analysis from the data collected from 169 participants who completed all the surveys in the registry. This is the first global Natural History Study addressing Chondrosarcoma. For the past nine weeks we have been sharing our findings (one result at a time) to help both the sarcoma / oncology medical profession and the CS patient community understand and use these results towards developing better protocols, practices / interventions as well as better research and clinical trials.
For chondrosarcoma patients, caregivers and families, we want them to be aware of their treatment options as well as the reported effectiveness of their treatment options. This is data collected as self-reports from fellow chondrosarcoma patients and caregivers who are keenly aware of their health and well-being. We are grateful to all who have shared their stories and those who choose to participate in the registry and we encourage patients diagnosed with chondrosarcoma to share their journey and help us create an aggravate database to help navigate research, clinical trials and medical practice.
We are learning a lot from our first analysis. We have looked at the need for CS patients to fully understand their Chondrosarcoma sub-type, address misdiagnosis, recurrence, metastasis, and examine the outcomes of surgery, radiation, traditional chemotherapy and immunotherapy. If you have not seen our earlier posts, we invite you to go to our web site: https://csfshayna.org/cs-foundation-blog/
Unfortunately, we had a small response from Chondrosarcoma patients who are either prescribed other medications or are enrolled in clinical trials with experimental drugs being used to treat chondrosarcoma.
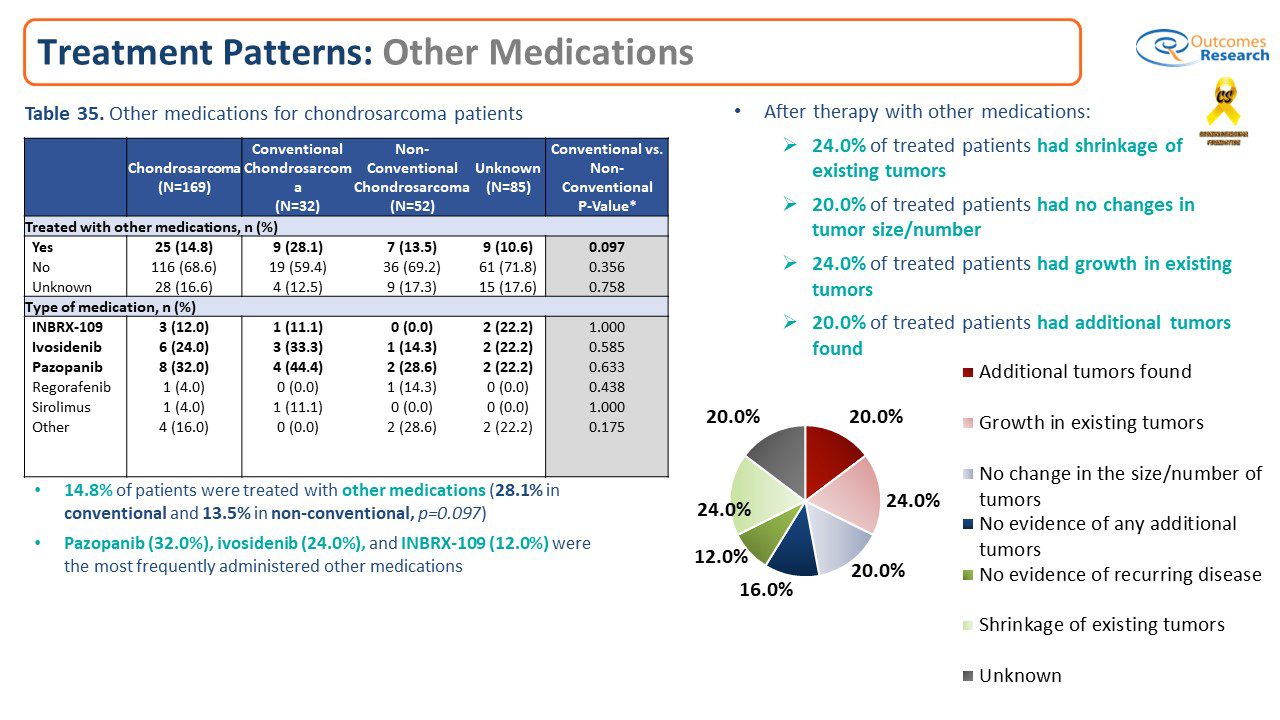
Results: A total of 25 patients reported that they were prescribed other medications after surgical intervention. A total of 14.8% of patients were treated with other medications 28.1% (n=9) in conventional and 13.5% (n=7) in non-conventional, and 10.6% (n=9) for those reporting they did not know their sub-type. Pazopanib (32.0%), Ivosidenib (24.0%), and INBRX-109 (12.0%) were the most frequently administered other medications. Results were mixed with 24.0% (n=6) of treated patients reporting had growth in existing tumors, and 20.0% (n=4) of treated patients reporting they had additional tumors found. In contrast, 20.0% (n=5) of treated patients reported they had no changes in tumor size/number and 24.0% (n=6) of treated patients had shrinkage of existing tumors.
Conclusions: There is no doubt that the first full data analysis of the chondrosarcoma patient registry revealed a low response from patients who were prescribed or were enrolled in a clinical trial. Ivosidenib 24.0% and INBRX-109 12.0% or Pazopanib (32.0%) were most prescribed other medications followed by Regorafenib 4%, Sirolimus 4% or other medications 16%. We need to reach out to chondrosarcoma patients who are enrolled in clinical trials and/or prescribed medications post surgically to treat chondrosarcoma. At this time the results are mixed, and no conclusions can be determined.
Your participation is essential.
Be A Part of the Chondrosarcoma Patient Registry.
To Register: https://chondrosarcoma.iamrare.org/
It is not too late to sign up to participate in the Chondrosarcoma Patient Registry. The register is open at any time for CS Patients or Caregivers to go on-line and complete 9 surveys which will take less than an hour to complete. We need you to share your journey and help progress research in chondrosarcoma.
The Chondrosarcoma Patient Registry creates a platform for patients around the world to strengthen their voices and share information about this rare bone cancer. This global resource will provide data for researchers to use to advance drug development and treatment options and help improve patient care. The patient information that the registry collects will help give direction to scientists on areas in which to focus their research efforts, and to hopefully allow clinicians to see trends in patient responses to treatment for chondrosarcoma. The hope for the future is to illuminate treatments that have had some success that may become the standard of care for this Ultra-rare bone cancer.