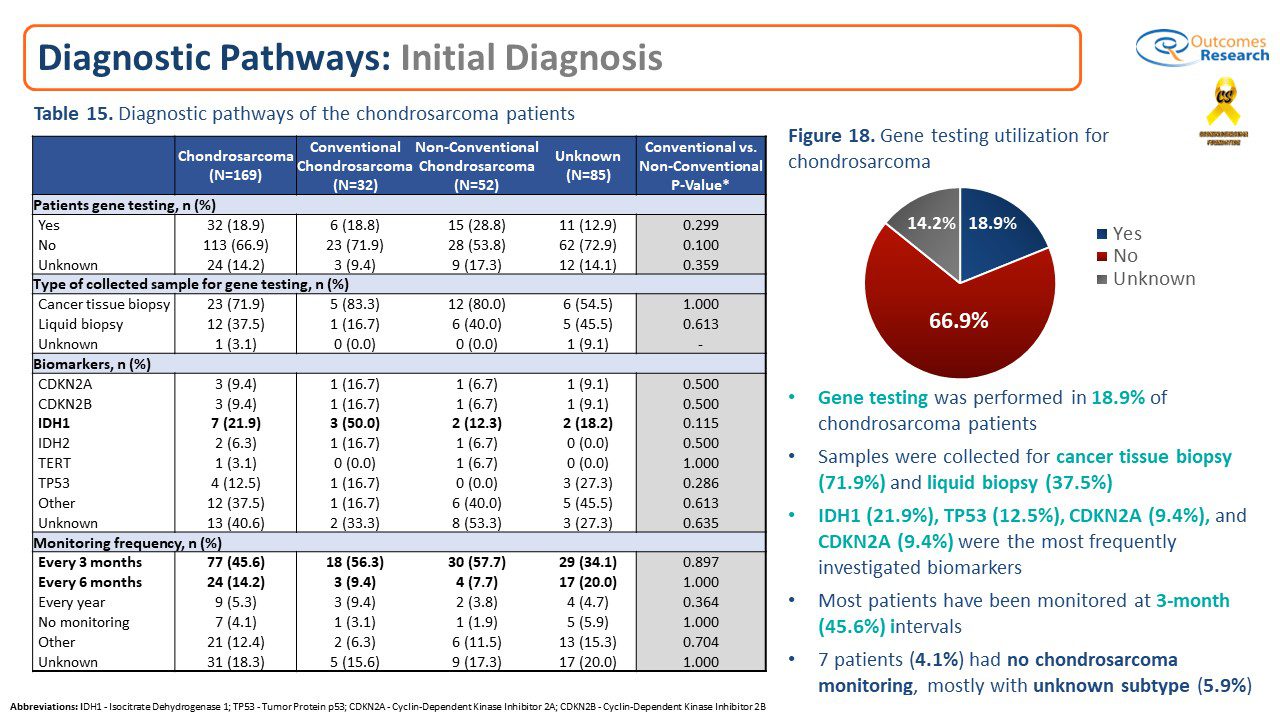
Chondrosarcoma Patient Registry reveals only a few Patients have had Gene testing
The ChondrosarcomaFoundation was the first foundation to be inducted into the NORD IAMRARE Patient Registry 2.0 platform. Since our launch we have had over 230 CS Patients participate in the registry and we just completed our first full data analysis from the data collected from 169 participants who completed all of the surveys in the registry. This is the first global Natural History Study addressing Chondrosarcoma.
We are sharing major findings (one result at a time) to help both the sarcoma / oncology medical profession and the CS patient community understand and use these results towards building developing better practice / interventions as well as better research and clinical trials.
This is an on-going retrospective analysis of the Chondrosarcoma Patient Registry. The database includes demographic, medical history, diagnostic, clinical, genetic, and patient-reported measures collected from patients or their relatives worldwide (from 19 countries) via the customized survey (IAMRARE® program powered by the National Organization for Rare Diseases). Patients were stratified by sarcoma type in three cohorts – conventional CS (conventional grade 1-3 sub types), non-conventional CS (other sub types), and unknown CS (unspecified chondrosarcoma type). Outcomes were explored in the total sample and statistically compared between conventional and non-conventional CS.
Results on Genomic Sequencing:The Chondrosarcoma Patient Registry discovered that only a small groupobtained a genomic sequencing test.Gene testing was performed in only 18.9% of chondrosarcoma patients. Samples were collected for cancer tissue biopsy (71.9%) and liquid biopsy (37.5%).The most frequent biomarkers were IDH1 (21.9%), TP53 (12.5%), and CDKN2A (9.4%).
Conclusion: Genomic sequencing is a promising tool for expanding the treatment options for chondrosarcoma patients. Genomic sequencing technologies are capable of characterizing known and novel clinically relevant biomarkers, like IDH1/2, and is becoming a useful tool for drug development, facilitating drug target discovery, clinical trial patient selection, and clinical correlate analyses to improve the quality and longevity of life for chondrosarcoma patients. Every cancer has a unique genome, and understanding the genetic characteristics of chondrosarcoma can help fight it at its origin. From a research perspective, the new frontier of genomic sequencing and personalized medicine shows promise for more effective treatments and, hopefully, will contribute to finding a cure.We want to encourage chondrosarcoma patients and family members to discuss the value of genetic testing with their treating oncologist / physician and in consultation determine if genomic testing is an option for them.
Your participation is essential. Be A Part of the Chondrosarcoma Patient Registry.
To Register: https://chondrosarcoma.iamrare.org/
It is not too late to sign up to participate in the Chondrosarcoma Patient Registry. The register is open at any time for CS Patients or Caregivers to go on-line and complete 9 surveys which will take less than an hour to complete. We need you to share your journey and help progress research in chondrosarcoma.
The Chondrosarcoma Patient Registry creates a platform for patients around the world to strengthen their voices and share information about this rare bone cancer. This global resource will provide data for researchers to use to advance drug development and treatment options and help improve patient care. The patient information that the registry collects will help give direction to scientists on areas in which to focus their research efforts, and to hopefully allow clinicians to see trends in patient responses to treatment for chondrosarcoma. The hope for the future is to illuminate treatments that have had some success that may become the standard of care for this rare bone cancer.