Chondrosarcoma Patient Registry indicates Immunotherapy has mixed results
Chondrosarcoma Patient Registry indicates only a small group of Chondrosarcoma Patients had Immunotherapy with mixed results
The ChondrosarcomaFoundation was the first foundation to be inducted into the NORD IAMRARE Patient Registry 2.0 platform. Since our launch on March 1, 2023 we have had over 230 CS Patients participate in the registry and we just completed our first full data analysis from the data collected from 169 participants who completed all of the surveys in the registry. This is the first global Natural History Study addressing Chondrosarcoma. For the past six weeks we have been sharing major findings (one result at a time) to help both the sarcoma / oncology medical profession and the CS patient community understand and use these results towards developing better protocols, practices / interventions as well as better research and clinical trials.
For chondrosarcoma patients, caregivers and families, we want them to be aware of their treatment options as well as the reported effectiveness of those treatment options. This is data collected as self-reports from fellow chondrosarcoma patients and caregivers and they are keenly aware of their health and well-being through their journey. We are grateful to all who have shared their stories and those who choose to participate in the registry.
We have looked at the need for CS patients to fully understand their Chondrosarcoma sub-type,as well as address misdiagnosis, recurrence, metastasis, and benefits of surgery. Last week we addressed Radiation therapy and this week we examine Immunotherapy as a post surgical treatment intervention.
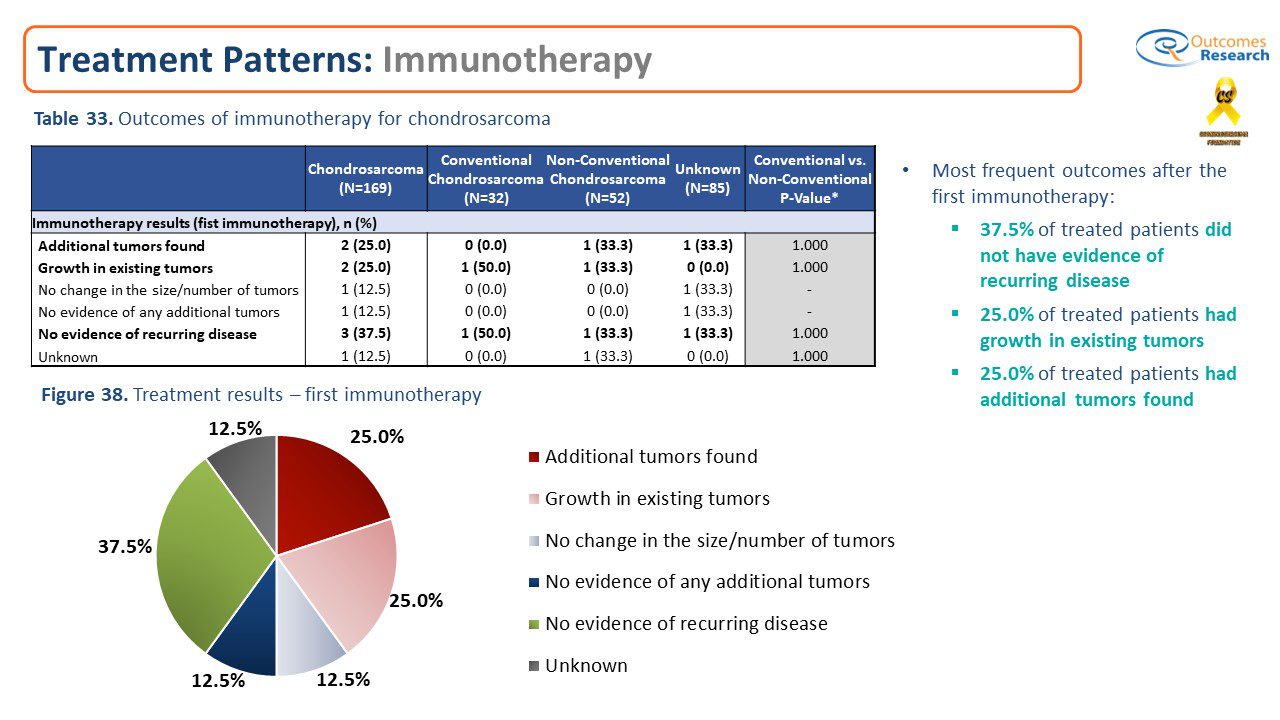
Results: It was reported that Immunotherapy was provided to only 4.7% (8 out of 169) of our patient group. It was noted that most immunotherapy-treated patients received 10-20 infusions (37.5%). The most common medications used for immunotherapy were pembrolizumab: keytruda (37.5%) and nivolumab (25.0%) and treatment-related side effects that caused immunotherapy cessation occurred in 25.0% of chondrosarcoma patients on immunotherapy. The most frequent outcomes after the first immunotherapy were not positive One quarter, 25.0% of treated patients had growth in existing tumors and another quarter 25.0% of treated patients had additional tumors found. On the positive side, 37.5% of treated patients did not have evidence of recurring disease.
Conclusions: It is very difficult to make any definitive conclusions about Immunotherapy because only a small group of chondrosarcoma patients reported being treated with it. According tothe Chondrosarcoma Patient Registry, the typical treatment regime of pembrolizumab and nivolumab was 10-20 infusions. It was also noteworthy and a consideration that Immunotherapy Treatment-related side effects caused immunotherapy cessation occurred in 25.0% of treated patients. One noted side effect was gastrointestinal infection and sepsis. Overall the results were mixed and inconclusive. More research and tracking through the Patient Registry is needed to determine whether immunotherapy is an effective tool in the treatment of chondrosarcoma.
Your participation is essential. Be A Part of the Chondrosarcoma Patient Registry.
To Register: https://chondrosarcoma.iamrare.org/
It is not too late to sign up to participate in the Chondrosarcoma Patient Registry. The register is open at any time for CS Patients or Caregivers to go on-line and complete 9 surveys which will take less than an hour to complete. We need you to share your journey and help progress research in chondrosarcoma. The Chondrosarcoma Patient Registry creates a platform for patients around the world to strengthen their voices and share information about this rare bone cancer. This global resource will provide data for researchers to use to advance drug development and treatment options and help improve patient care. The patient information that the registry collects will help give direction to scientists on areas in which to focus their research efforts, and to hopefully allow clinicians to see trends in patient responses to treatment for chondrosarcoma. The hope for the future is to illuminate treatments that have had some success that may become the standard of care for this rare bone cancer.